Software
FDA-compliant results documentation with Microscopy Imaging Software
Transferring microscopy applications into production often requires validation activities.

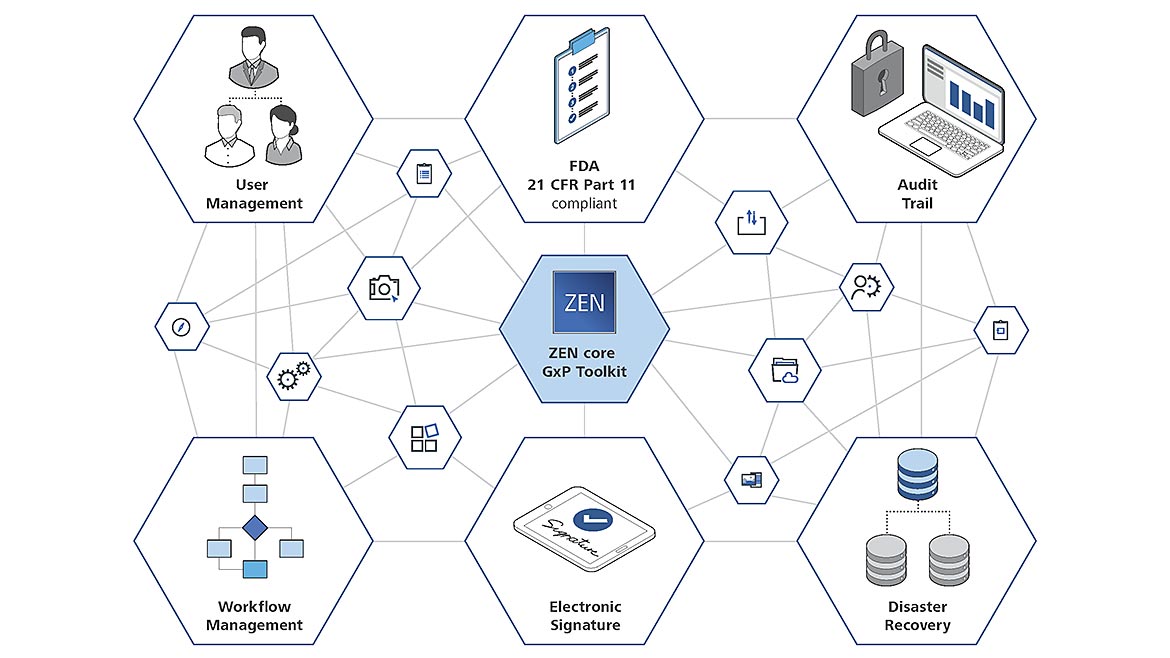
ZEISS ZEN core with GxP module offers the appropriate software for good practice microscopy in the, e.g., medical, pharmaceutical or aerospace industries. All parameters generated from the microscope are stored in the software. Source: ZEISS
The use of microscopic techniques for quality control and product development has been in practice for decades. When there were no digital cameras, the view through the microscope with the aid of an eyepiece micrometer was the way to obtain reliable measurement results – for example for the visual measurement of a particle.
The image documentation was complex because it was done on film in the darkroom. The result report was according to an inspection manual, signed and then carefully filed away to meet the prescribed retention periods. Under the keyword “a picture is worth a thousand words” is the image-based report of a microscopic examination, supplemented by measurement data, a common documentation medium for describing defects, quality documentation and laboratory data.
Expectations of data integrity, data security and reproducibility of the results, however, require more than simply storing of measurement data in a report. Regulatory requirements from the laboratory environment and the industry (e.g. FDA 21 CFR Part 11, GAMP5, EG GMP, etc.) also require in addition:
- User Management and Password Rules to make sure only users having the respective rights can access the system
- Electronic signatures to protect the recipes and result data from manipulation or modification
- Complete recording of all process-critical activities in an Audit Trail
- Versioning and release-procedures of recipes using 4-eyes principle
To meet these requirements, it is necessary to closely integrate the microscopy hardware into the control software, so that changes to the overall system can be recorded completely electronically and securely saved. The storage of the result data, procedures and users’ needs are to be carefully decided and as they might need to be backed up and stored for many years.
What to look for in software
A module that offers the appropriate software for good practice microscopy in the medical, pharmaceutical or aerospace industries, for example, is ideal. All parameters generated from the microscope are stored in the software. With integrated functions for user management, logging of work steps and versioning of task templates, electronic signature of results and data recovery are available in the event of a deviation of a workflow or even a system crash.
It’s beneficial for a software to enable fully validated microscopic examinations and perform them e.g. according to FDA 21 CFR Part 11. This guarantees traceability and reproducibility. The user administration provides a clear assignment of the employees registered in the system. The audit trail documents all work steps, both on the microscope as well as in the software supported evaluation. The audit trail records every step of a workflow and reports “Who” did “What,” “When” and “Why.”
Electronically signed measurement results and reports are safely stored in a multi-user client-server database, where company IT rules can be applied.
The module offers consistent user orientation for increasingly complex workflows. The software is characterized by its easy learnability and high ease of use. Each user is only provided with the functions and tools that are displayed on the screen. After a short introduction, laboratory managers are able to create and release clearly structured tasks that can then be reproducibly taken over by the employees.

Services for validation
Transferring microscopy applications into production often requires validation activities. All of the tasks and requirement needs to be described in a User Requirements Documentation (URS) document. Followed by a professional Installation Qualification (IQ), and Operational Qualification (OQ) as well as a training of the Users and Operators. In addition, if microscopes are used for measurement tasks, professional certified calibration is important.
Conclusion
Microscopy systems must ensure data integrity and process reliability to meet regulatory requirements such as FDA 21 CFR Part 11 with a software solution providing the respective functionalities such as Audit Trail, Electronic Signatures and User Management. Central microscopy applications in quality inspection and product development become validatable and more secure. Installation Qualification (IQ) and Operational Qualification (OQ) must be performed to bring those microscopes into production. Both existing tasks as well as new requirements can be seamlessly integrated into the digital workflow. The solution must support users in concentrating on their actual laboratory tasks while the necessary validation and protocol functions are performed automatically in the background. In combination with this software solution, modern microscopy systems guarantee objective results.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





